JESEI
teacher’s notes
student’s notes




Carbon cycle in the lab: carbon products and the processes that link them
(teacher’s notes)
This material is designed for students aged 12 to 14 (years 8 and 9).
Topic
This activity is one of five aimed to teach students about the nature of carbon, the different types of
compounds it exists in (eg charcoal, glucose, carbon dioxide), the biochemical reactions it takes part in
(photosynthesis and respiration), the range of processes that carbon and carbon compounds are involved in on
Earth, and how these link together form the carbon cycle.
The other activities are
Carbon cycle: where is this crucial carbon?; a teacher-led discussion interspersed with demonstrations in which
the teacher burns a range of materials over a Bunsen flame, forming charcoal, to illustrate that they contain
carbon.
Cycling carbon: seeing how plants use carbon dioxide in the lab; a short pupil practical exploring the uptake of
carbon dioxide from the atmosphere by plants for photosynthesis.
Carbon cycle: releasing dinosaur breath in the lab; a short pupil practical exploring the storage of carbon in the
fossils that make up limestone and chalk.
Carbon cycle: exchanging carbon dioxide between the atmosphere and ocean; a short pupil practical comparing
how well carbon dioxide dissolves in sea water compared with fresh water.
Context
An understanding of the carbon cycle is essential to the debate about global warming, an environmental issue
that most students will have heard about. Since the Earth’s atmosphere formed, it seems to have always
contained carbon dioxide in varying amounts Carbon dioxide is a ‘greenhouse gas’ through which light radiation
can pass but which absorbs some of the heat radiation produced by light irradiating the Earth’s surface. This
causes the Earth’s surface and atmosphere to be warmer than it would otherwise be and without the
‘greenhouse effect’ the Earth would probably be completely frozen. Humans, as all life on Earth, have always
been part of the carbon cycle, but now (since the industrial revolution) the large scale burning of oil, coal and
natural gas, along with deforestation, is leading to increasing atmospheric carbon dioxide levels. This in turn is
related to an enhanced greenhouse effect and consequent climatic change. An understanding of the factors
affecting global warming leads to an understanding of the measures required to reduce their impact. This can
link into economic and political debates on the subject. Students should have already covered photosynthesis
and respiration in order to do this set of activities effectively.
Teaching points
The carbon cycle is a “big idea” that can be difficult to understand because parts of it work at a microscopic
scale while other parts affect the whole Earth. Also, some parts happen in milliseconds whilst others can take
millions of years to have an effect. We can get a feel for how the whole carbon cycle works by turning the lab.
into a model of the carbon cycle and seeing how the different things that are produced in the cycle (the
products) fit together with the way those products are made (the processes).
Apparatus and materials
The teacher will need:
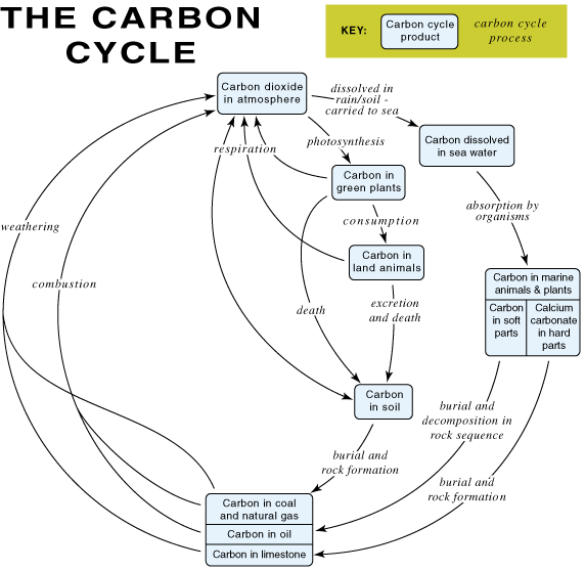
An OHT showing the carbon cycle (Figure 1).
A copy of the carbon cycle A4 sheet for each pupil (Figure 1).
Either a copy of Carbon cycle in the lab: carbon products and the processes that link them (for pupils)
worksheet for each pupil or an OHT version
1 set of A4 sheets of ‘products’ (Download in Word format or Download in Portable Document Format (PDF)),
(Carbon dioxide in the atmosphere, Carbon in green plants; Carbon in soil; Carbon in marine animals and
plants/ Carbon in soft parts/ Calcium carbonate in hard parts; Carbon in coal and natural gas/ Carbon in oil/
Carbon in limestone; Carbon dissolved in seawater)
1 set of A4 sheets of ‘processes’ (Download in Word format or Download in Portable Document Format (PDF)),
(dissolved in rain/ soil water - carried to sea; absorption by organisms; burial and rock formation; burial and
decomposition in rock sequence; burial and rock formation; weathering; combustion; photosynthesis;
consumption; death; excretion and death; respiration)
Set of specimens of ‘products’:
Carbon dioxide in the atmosphere – a stoppered test tube, labelled ‘Atmosphere’
Carbon in green plants - grass or leaf (or a picture)
Carbon in land animals - mirror
Carbon in soil - bag of soil
Carbon in seawater - test-tube of water labelled ‘seawater’
Carbon in marine animals and plants- sea shells
Carbon in coal and natural gas - coal
Carbon in oil – a stoppered, labelled test tube of crude oil (treacle could be used as a substitute if crude oil is
not available)
Carbon in limestone - limestone
To prepare:
A4 sheets of the ‘products’ of the cycle are put in a rough circle on desks around the room.
Keep the A4 sheets of the ‘processes’ for use later.
Safety
Be aware of the risk of breaking test-tubes.
Activity
Through discussion with the class, the teacher puts the specimens in the correct places on the carbon cycle
product sheets laid out around the room.
Then, again through interactive discussion, the teacher puts the ‘processes’ sheets in the correct places between
the products around the room.
Finally the pupils are shown an OHT version of the cycle and are given their own copies. They should write the
examples of the ‘products’ they have been shown in the correct places on their version of the diagram. Faster
pupils can then answer the questions on the worksheet.
Questions
Which products of the carbon cycle:
Q 1. can you see out of the window? = Green plants, land animals, soil, limestone.
Q 2. can you never see? = Carbon dioxide in the atmosphere and dissolved in seawater, natural gas.
Q 3. might you see in a quarry? = Limestone, coal, soil, plants, land animals, the hard parts of marine animals.
Q 4. are fluids (liquids or gases)? = Carbon dioxide in the atmosphere, natural gas, oil, dissolved carbon
dioxide in seawater.
Q 5. last the longest? = Limestone, coal, oil/natural gas trapped in rocks, carbon dioxide in the atmosphere.
Q 6. might affect global warming? (This is for the more able/interested and is a ‘warm up’ for activities to
come!) = Greenhouse gases carbon dioxide and methane (natural gas); coal. oil, natural gas that will produce
carbon dioxide when burned, green plants that remove CO2 in photosynthesis, animals and plants that release
CO2 in respiration (all the other products may affect global warming too ,eg weathering of limestone releases
more CO2, when there is more CO2, plant life is more prolific and the animal life feeding on the plant life is
more prolific, etc).
Figure 1 The carbon cycle

teacher’s notes
student’s notes




Carbon cycle in the lab:
carbon products and the
processes that link them
(teacher’s notes)
This material is designed for students aged 12 to 14
(years 8 and 9).
Topic
This activity is one of five aimed to teach students about
the nature of carbon, the different types of compounds
it exists in (eg charcoal, glucose, carbon dioxide), the
biochemical reactions it takes part in (photosynthesis
and respiration), the range of processes that carbon and
carbon compounds are involved in on Earth, and how
these link together form the carbon cycle.
The other activities are
Carbon cycle: where is this crucial carbon?; a teacher-led
discussion interspersed with demonstrations in which
the teacher burns a range of materials over a Bunsen
flame, forming charcoal, to illustrate that they contain
carbon.
Cycling carbon: seeing how plants use carbon dioxide in
the lab; a short pupil practical exploring the uptake of
carbon dioxide from the atmosphere by plants for
photosynthesis.
Carbon cycle: releasing dinosaur breath in the lab; a
short pupil practical exploring the storage of carbon in
the fossils that make up limestone and chalk.
Carbon cycle: exchanging carbon dioxide between the
atmosphere and ocean; a short pupil practical
comparing how well carbon dioxide dissolves in sea
water compared with fresh water.
Context
An understanding of the carbon cycle is essential to the
debate about global warming, an environmental issue
that most students will have heard about. Since the
Earth’s atmosphere formed, it seems to have always
contained carbon dioxide in varying amounts Carbon
dioxide is a ‘greenhouse gas’ through which light
radiation can pass but which absorbs some of the heat
radiation produced by light irradiating the Earth’s
surface. This causes the Earth’s surface and atmosphere
to be warmer than it would otherwise be and without
the ‘greenhouse effect’ the Earth would probably be
completely frozen. Humans, as all life on Earth, have
always been part of the carbon cycle, but now (since the
industrial revolution) the large scale burning of oil, coal
and natural gas, along with deforestation, is leading to
increasing atmospheric carbon dioxide levels. This in
turn is related to an enhanced greenhouse effect and
consequent climatic change. An understanding of the
factors affecting global warming leads to an
understanding of the measures required to reduce their
impact. This can link into economic and political debates
on the subject. Students should have already covered
photosynthesis and respiration in order to do this set of
activities effectively.
Teaching points
The carbon cycle is a “big idea” that can be difficult to
understand because parts of it work at a microscopic
scale while other parts affect the whole Earth. Also,
some parts happen in milliseconds whilst others can
take millions of years to have an effect. We can get a feel
for how the whole carbon cycle works by turning the lab.
into a model of the carbon cycle and seeing how the
different things that are produced in the cycle (the
products) fit together with the way those products are
made (the processes).
Apparatus and materials
The teacher will need:
An OHT showing the carbon cycle (Figure 1).
A copy of the carbon cycle A4 sheet for each pupil
(Figure 1).
Either a copy of Carbon cycle in the lab: carbon products
and the processes that link them (for pupils) worksheet
for each pupil or an OHT version
1 set of A4 sheets of ‘products’ (Download in Word
format or Download in Portable Document Format
(PDF)), (Carbon dioxide in the atmosphere, Carbon in
green plants; Carbon in soil; Carbon in marine animals
and plants/ Carbon in soft parts/ Calcium carbonate in
hard parts; Carbon in coal and natural gas/ Carbon in
oil/ Carbon in limestone; Carbon dissolved in seawater)
1 set of A4 sheets of ‘processes’ (Download in Word
format or Download in Portable Document Format
(PDF)), (dissolved in rain/ soil water - carried to sea;
absorption by organisms; burial and rock formation;
burial and decomposition in rock sequence; burial and
rock formation; weathering; combustion;
photosynthesis; consumption; death; excretion and
death; respiration)
Set of specimens of ‘products’:
Carbon dioxide in the atmosphere – a stoppered test
tube, labelled ‘Atmosphere’
Carbon in green plants - grass or leaf (or a picture)
Carbon in land animals - mirror
Carbon in soil - bag of soil
Carbon in seawater - test-tube of water labelled
‘seawater’
Carbon in marine animals and plants- sea shells
Carbon in coal and natural gas - coal
Carbon in oil – a stoppered, labelled test tube of crude
oil (treacle could be used as a substitute if crude oil is
not available)
Carbon in limestone - limestone
To prepare:
A4 sheets of the ‘products’ of the cycle are put in a rough
circle on desks around the room.
Keep the A4 sheets of the ‘processes’ for use later.
Safety
Be aware of the risk of breaking test-tubes.
Activity
Through discussion with the class, the teacher puts the
specimens in the correct places on the carbon cycle
product sheets laid out around the room.
Then, again through interactive discussion, the teacher
puts the ‘processes’ sheets in the correct places between
the products around the room.
Finally the pupils are shown an OHT version of the cycle
and are given their own copies. They should write the
examples of the ‘products’ they have been shown in the
correct places on their version of the diagram. Faster
pupils can then answer the questions on the worksheet.
Questions
Which products of the carbon cycle:
Q 1. can you see out of the window? = Green plants,
land animals, soil, limestone.
Q 2. can you never see? = Carbon dioxide in the
atmosphere and dissolved in seawater, natural gas.
Q 3. might you see in a quarry? = Limestone, coal, soil,
plants, land animals, the hard parts of marine animals.
Q 4. are fluids (liquids or gases)? = Carbon dioxide in
the atmosphere, natural gas, oil, dissolved carbon
dioxide in seawater.
Q 5. last the longest? = Limestone, coal, oil/natural gas
trapped in rocks, carbon dioxide in the atmosphere.
Q 6. might affect global warming? (This is for the more
able/interested and is a ‘warm up’ for activities to come!)
= Greenhouse gases carbon dioxide and methane
(natural gas); coal. oil, natural gas that will produce
carbon dioxide when burned, green plants that remove
CO2 in photosynthesis, animals and plants that release
CO2 in respiration (all the other products may affect
global warming too ,eg weathering of limestone releases
more CO2, when there is more CO2, plant life is more
prolific and the animal life feeding on the plant life is
more prolific, etc).
Figure 1 The carbon cycle


- Home
- contents
- help
- glossary
- Magnetic patterns 1
- Magnetic patterns 2
- Mantle convection
- Metamorphics
- Minerals & elements
- Plate riding
- Plate tectonic story
- Protecting the earth
- Rock cycle in lab
- Sedimentary rocks
- Separating mixtures
- Sequencing of rocks
- Solid mantle
- Structure of earth 1
- Structure of earth 2
- Structure of earth 3
- Tree rings
- Weathering
- Gravestones
- Lab volcano
- Investigate earth